By TIMOTHY W. MARTIN
OxyContin is set to go off patent next year, but the maker of the powerful and heavily abused prescription painkiller is trying to extend its exclusive rights to the drug, arguing that a new version it spent $100 million to develop might substantially curtail abuse.
Whether Purdue Pharma LP will be able to protect its reformulated OxyContin will be decided by the courts. The company has 16 patent-infringement lawsuits pending against 10 generic-drug manufacturers, including Watson Pharmaceuticals Inc. and Teva Pharmaceutical Industries Ltd.
The Stamford, Conn.-based company says the new version of OxyContin it introduced in 2010, and which has patent protection until 2025, makes it tougher to abuse than the original formulation, whose patent expires next year. Generic-drug makers should be prevented from producing the original version of OxyContin, it says.
"We believe that no generics to OxyContin should be approved that are not formulated to be abuse-deterrent," says a spokesman for the company, whose OxyContin sales reached more than $2.8 billion last year.
Manufacturers of generic drugs say Purdue Pharma is just trying to protect a lucrative market for itself, and that they can make their own abuse-deterrent formulations of the drug.
Even if the company loses its legal battles, the Food and Drug Administration may still stave off generic versions of the drug. The FDA is reviewing what reformulations or safeguards it should require generic OxyContin to contain, and is expected to rule by the end of this year.
"We need to make it possible to make these abuse-deterrent formulations," Douglas D. Throckmorton, a deputy director in the FDA's Center for Drug Evaluation and Research, said in an interview.
The agency's decision could have wide-ranging implications for the nation's war on drugs. Painkillers caused more deaths in the U.S. in 2011 than heroin and cocaine combined, and drug and law-enforcement officials are struggling to figure out how to contain illegal trafficking in the legal medications.
Complicating the matter for the FDA, however, is the fact that little research has been done to show whether the safeguards like those Purdue Pharma has patented are effective in thwarting abuse.
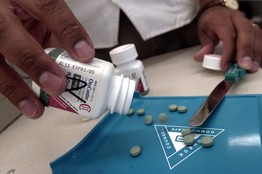
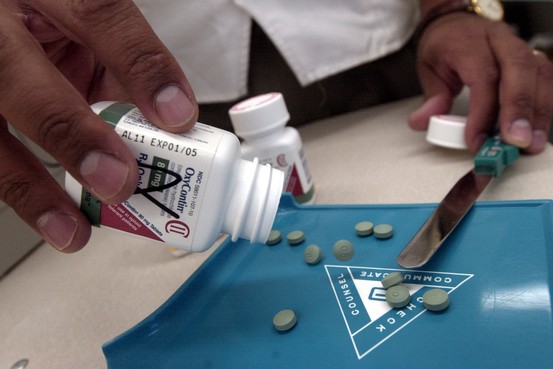
OxyContin abusers typically heated or crushed the original pills, then injected, snorted or smoked their contents, for a heroinlike high. The semisynthetic opiate stimulates nerve receptors to block pain and produces a feeling of euphoria.
The new version of the pill is infused with polyethylene oxide, a polymer that makes the pill tough to crush for snorting or to heat for intravenous injection. The company also altered the formula so that the pill's powdery contents turn into a jellylike substance if water is added to make a solution for an injection. Users can still get high from the pills if they are willing to swallow enough of them or engage in more elaborate processing.
In past years the company has faced lawsuits from federal and state officials alleging it deceptively marketed OxyContin as being less addictive and less prone to abuse than other painkillers, because of the drug's extended-release formula. The lawsuits alleged that this marketing made doctors more comfortable prescribing OxyContin and contributed to an upsurge in painkiller abuse in the past decade.
In 2007, Purdue Pharma pleaded guilty in federal court in Virginia to criminal charges of misbranding the drug with the intent to defraud and mislead the public about its addictive qualities. The company and three top executives paid a $634 million fine. The same year, Purdue agreed to a $20 million settlement of similar allegations with 27 state attorneys general.
In connection with the settlement with the Justice Department, Purdue acknowledged that some sales representatives and managers made misstatements in the promotion of OxyContin in violation of Purdue policies that overstated benefits and understated risks.
OxyContin was unlike nearly every other painkiller when it hit the market in 1995. Its extended-release mechanism meant patients in searing pain could take just one pill every 12 hours. Few other widely-prescribed oral painkillers approach the strength of OxyContin's 80-milligram tablet—more than double the dosage of most opioid painkillers.
As evidence the new formulation is cutting down on abuse, the company cited a report the FDA required it to compile and submit. The report, citing preliminary data, says that the street price of OxyContin—an indication of demand—fell to 65 cents per milligram in the second quarter of 2011, a 22% decline from its 81-cent price in the first quarter of 2010, based on a survey of 300 law-enforcement officials in all 50 states.
The number of visits to poison-control centers and admissions to addiction-treatment programs attributed to OxyContin also showed declines after the reformulation, according to the report, part of a number of continuing studies.
The Generic Pharmaceutical Association, a trade group for generic-drug makers, questions the effectiveness of Purdue's new formulation, pointing to the lack of independent evidence. Regulators and doctors should decide, it argues.
Other painkiller makers have reformulated their drugs. Endo Pharmaceuticals Inc. introduced an updated version of its Opana this year that is more difficult to crush and inject. Pfizer Inc. and Acura Pharmaceuticals Inc. introduced an abuse-deterrent version of their Oxecta painkiller last year.
"Who is saying this reformulation is safer?" asks Shawn Brown, the association's vice president of state affairs.
A single 80-milligram tablet of OxyContin retails for around $12 or more when obtained legally, according to state Medicaid and other data. It is difficult to predict how much a legally obtained generic version would cost, though generics are typically 30% to 80% cheaper than the branded version, the trade group says.
Other industry experts say that generic manufacturers have the technological capability to produce their own safeguards such as those introduced and patented by Purdue Pharma.
Watson declined to comment, citing its litigation with Purdue Pharma. Teva believes it has the capability to produce an equivalent version of OxyContin's new formulation, though it depends on what the FDA decides to require, says David M. Stark, general counsel for Teva's American operations. The company declined to elaborate.
Branded-drug makers have sparred with generics makers before over product alterations. Last October, Warner Chilcott PLC, an Irish drug maker, tried to ward off a generic version of its Doryx severe acne medication by adding a line across the top of the tablets to make them easier to be divided into thirds. A U.S. District Court in New Jersey ruled in April that the change didn't merit extending the company's patent protection.
OxyContin's falling street price is a sign that demand is in decline and the reformulation is working at thwarting abuse, says John Burke, president of the National Association of Drug Diversion Investigators, a nonprofit group that works with police, physicians, states and manufacturers to curb drug abuse.
"Addicts want that rush into their body—that immediate effect," says Mr. Burke. With the reformulation, it takes swallowing three or four OxyContin pills to get the same high as before, he added. Some unusual ways include multistep processes of microwaving and freezing pills, as well as meticulously filing them down to powder.
Write to Timothy W. Martin at timothy.martin@wsj.com
A version of this article appeared June 28, 2012, on page A1 in the U.S. edition of The Wall Street Journal, with the headline: OxyContin Maker Guards Exclusivity.








![[image]](https://iza-server.uibk.ac.at/pywb/dilimag/20210113092156im_/http://si.wsj.net/public/resources/images/OB-TP243_wynn07_A_20120702000014.jpg)
![[image]](https://iza-server.uibk.ac.at/pywb/dilimag/20210113092156im_/http://si.wsj.net/public/resources/images/MK-BV389_HOUGHT_C_20120701170025.jpg)
![[image]](https://iza-server.uibk.ac.at/pywb/dilimag/20210113092156im_/http://si.wsj.net/public/resources/images/MI-BP829_curry_C_20120701170605.jpg)
![[image]](https://iza-server.uibk.ac.at/pywb/dilimag/20210113092156im_/http://si.wsj.net/public/resources/images/NY-BT061_SWIM_C_20120701193212.jpg)
![[image]](https://iza-server.uibk.ac.at/pywb/dilimag/20210113092156im_/http://si.wsj.net/public/resources/images/P1-BG850_SCYTHE_C_20120629173254.jpg)
![[image]](https://iza-server.uibk.ac.at/pywb/dilimag/20210113092156im_/http://si.wsj.net/public/resources/images/OB-TO602_boxila_C_20120628203443.jpg)






Most Recommended
“Try and fathom the hypocrisy of ...;”
“Perhaps, Mr Gensert, it's not th...;”
“Another thoughtful and concise...;”
“However, Ms Napolitano immediate...;”
“My thought, exactly...You go on...;”